The CE sanitary certificate of product registration is a mandatory requirement to health market products in Europe. A product with health CE certificate is easily identified by a label and instructions for use by the CE symbol + serial number. For example:
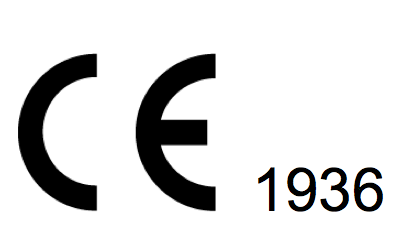
If a Notified Organization is involved in the evaluation of conformity of the product the health CE comes with the identification number of the product. (In the picture: 1936 is the identification number Notified by TÜV).
In the market, the CE shows that the product fulfills with the directives and legislation that are recommended. In Europe is illegal the sale and subsequent use of products that do not have this certificate, along with the dangers they pose to health. All sanitary products must have a CE sanitary certificate and put it at the disposal of the customers.
CE sanitary certificate as a guarantee
The directives require a minimum requirements sanitary CE for proper evaluation and certification of products. The law requires all manufacturers to provide the following guarantees:
- Information Guarantee: There must be a technical product documentation called Technical File. This includes specifications, plans, user manual, technical assistance manuals and accompanying documentation.
- Security Guarantee: a product demonstration must be made to ensure is in accordance with the rules applied to it, according to their classification.
- Guarantee of compliance with specifications and due efficacy: Clinical and, in EPTE® percutaneous electrolysis therapy case, Clinical for proper evaluation of safety and compliance with product specifications.
- Guarantee of Quality: The company must implement a system to ensure the quality according to ISO 13485 with the corresponding monitoring and ensuring their correct implementation.
Sanitary Certificate CE recognition process
Every product that you want to commercialize as a sanitary product, must follow a process in order to be recognized as a sanitary product and for it to obtain the CE sanitary certificate and then be sell:
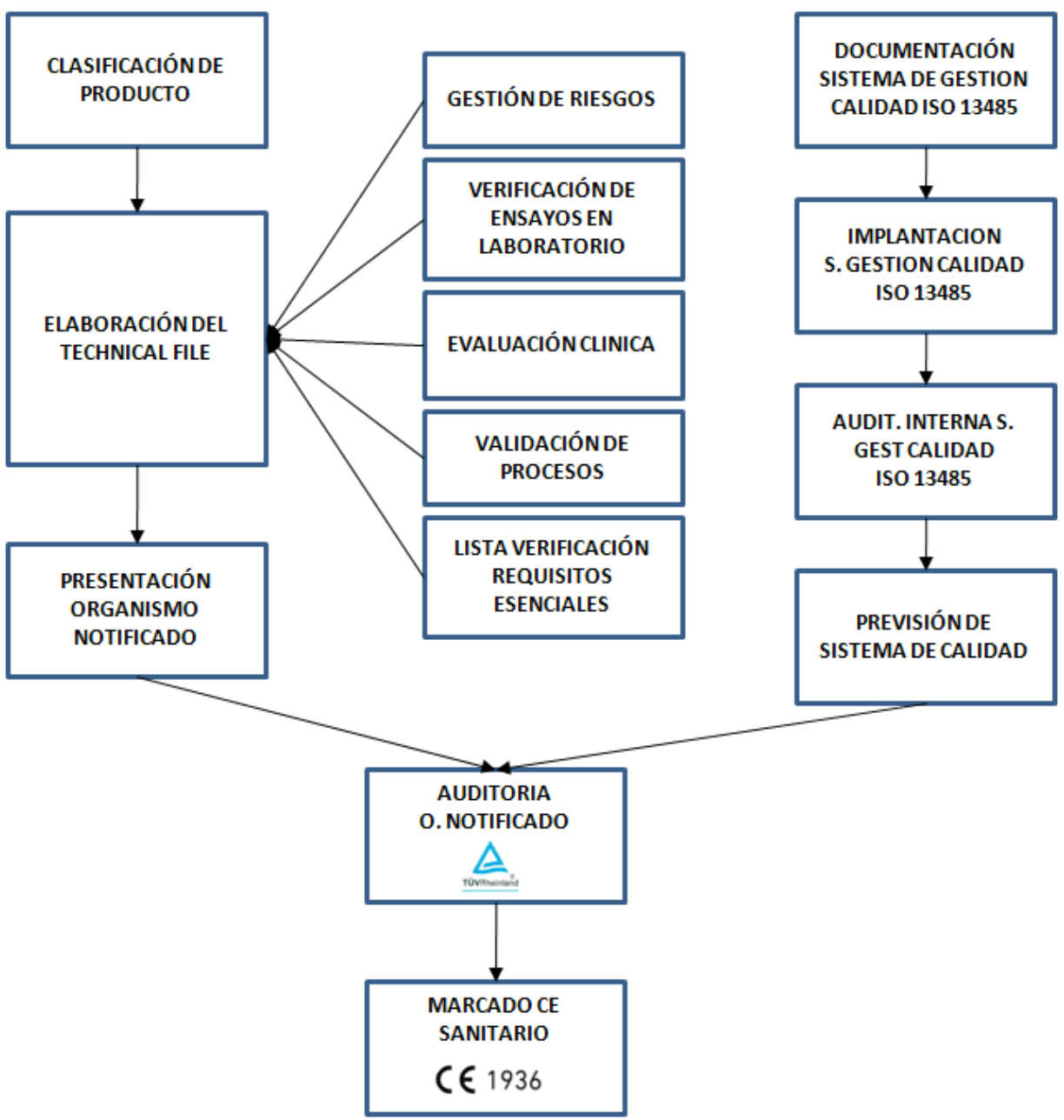
After the product design, it is necessary to create a documentation to ensure compliance with all applicable laws (Technical File), whose level of demand depends on their classification.The law applied is the UNE-EN – 60601-1: 2008. + Correction: 2010 for electromedical equipments or the international equivalent that is IEC 60601-1: 2005+ race: 2006. The generated documentation is:
- Risk management document;
- Document of the validation processes as manufacture, use aptitude, software verification, etc.
- Compliance verification of essential requirements in accordance with the law in force.
After this, a verification is made by an accredited laboratory to prove that the device respects all requirements to be considered as a sanitary product:
a) Immunity Tests:
- Electrostatic discharge;
- Immunity to conducted disturbances, induced by RF radio frequency fields;
- Magnetic field industrial reference.
b) Testing emission (radiation emission)
c) Electrical Safety:
- Identification and brand;
- Protection against electrical dangers;
- Protection against mechanical dangers;
- Hazards protection from unwanted and excessive radiation;
- Hazards protection against excessive temperatures and other hazards;
- Precision controls, instruments and protection against hazardous outputs;
- dangerous situations and fault conditions;
- Construction of the product.
Once the conformity is obtained, the CE mark is given. This CE mark is still not the CE sanitary certificate. The process to obtain the CE sanitary certificate has some more steps:
Get the CE sanitary certificate: last steps

- Clinical trial for the CE sanitary certificate;
- Contribution of real clinical trials with a valid sample, the experimental group, the control group, authorized test and signed by an ethics committee;
- The trial evaluates the device has health benefits.
- Once all these steps are completed, the documentation has to be delivered to a Notified Organism authorized by the European Community and it has to verify and validate the documentation.
In addition to this the Notified Organism must audit the quality management system in the manufacturing, storage and distribution process according to ISO 13485. “This International Standard specifies requirements for a quality management system that can be used by a organization for the design and development, production, installation and servicing of medical devices and for the design, development and provision of related services.
Finally, after a favorable audit of the Notified Organism, the CE sanitary certificate is obtained.
In Europe only medical devices with the CE sanitary certificate can be sell.
The CE sanitary certificate is obligatory and authorities can sanction breaches related to the CE marking of products.

